OnKO•3T-ORL Leader: Sophie MARTIN
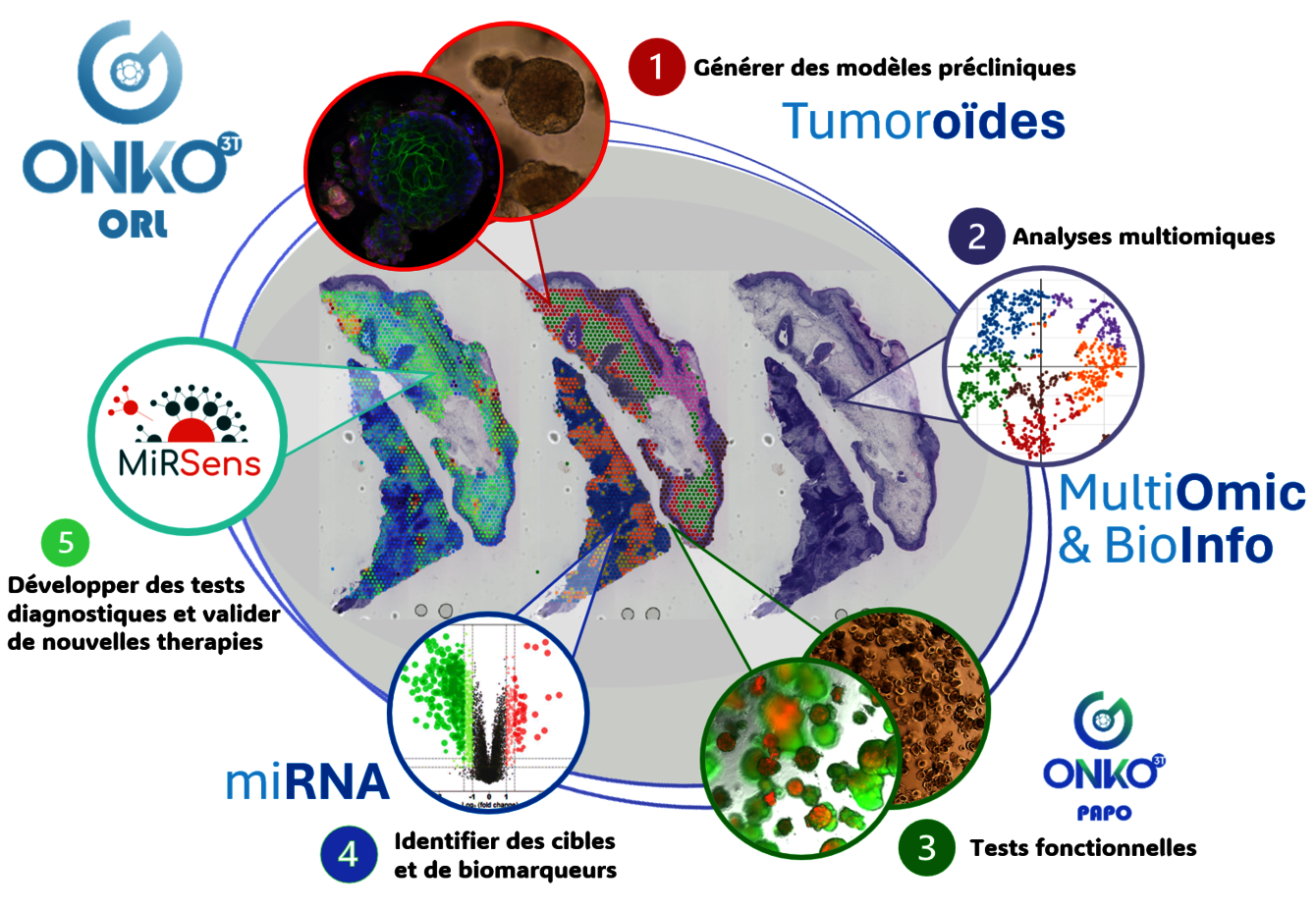
OnKO•3T-ORL is investigating the involvement of different players (caveolin-1, small non-coding RNAs or miRNAs, p53, ΔNP63) in the development, progression, relapse and radio-chemoresistance of head and neck cancers (cancers of the mouth, pharynx and larynx) linked or not to human papillomavirus (HPV) infection.
One of our priority topics is the involvement of small non-coding RNAs or miRNAs, miR-30-3p, in the local or systemic progression of HPV-negative head and neck cancers and their value as biomarkers predicting relapse. The identification of the onco-suppressive (via suppression of TGFβ/BMP, Conrad et al., 2023) and immunomodulatory (via induction of PD-L1/PD-1/proinflammatory cytokines, Conrad et al, in preparation, 2024) activity of miR-30-3p prompts us to investigate their potential as therapeutic tools, whether single or combined, targeting the immune microenvironment. To address these issues, we are developing 3D patient-derived models of simple or immunocompetent tumour organoids, which may or may not be integrated into microfluidic systems (tumouroid-on-a-chip development). In collaboration with ICANS (IPRICE study) and BrightSens Diagnostics, we are working on the value of miRs as predictive markers of response to immunotherapies by participating in an ancillary study of the IPRCE clinical trial (Carinato et al., 2023).