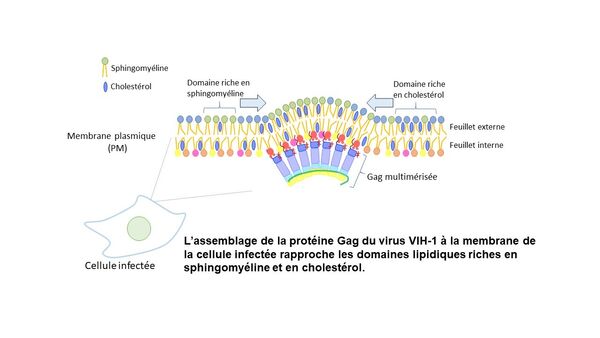
Viral Gag protein plays a central role in virus budding. Here, we report the interaction between Gag and host cell lipids using different quantitative and super-resolution microscopy techniques in combination with specific probes that bind endogenous lipids. Our results indicate that the binding of Gag to PM restricts the mobility of and enlarges specific lipid domains enriched with sphingomyelin but not cholesterol. Moreover, Gag multimerization induces sphingomyelin-rich and cholesterol-rich lipid domains to be in close proximity in a curvature-dependent manner.
Our results highlight the importance of host cell lipids in the formation of HIV-1 and perhaps also other envelope viruses such as COVID-19. Our results also raise the possibility of targeting host cell lipids to attach envelope viruses.
Tomishige, N. ; Bin Nasim, M. ; Murate, M. ; Pollet, B. ; Didier, P. ; Godet, J. ; Richert, L. ; Sako, Y. ; Mély, Y. & Kobayashi, T. HIV-1 Gag targeting to the plasma membrane reorganizes sphingomyelin-rich and cholesterol-rich lipid domains Nature Communications volume 14, Article number: 7353 (2023)