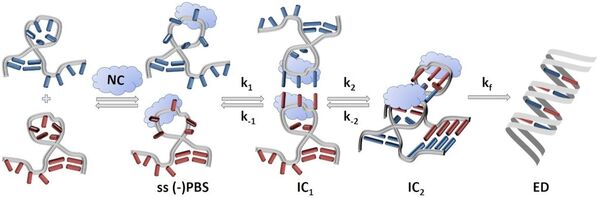
Interconversions between nucleic acid structures, which are frequently promoted by nucleic acid chaperone proteins, play an important role in biology. FRET is widely used to monitor these interconversions, mainly through stopped-flow experiments that only provide a weighted average of the emission of all species in solution. In collaboration with the team of J. Leonard (IPCMS, Strasbourg), this limitation was lifted by combining time-resolved fluorescence (TRF) with droplet microfluidics (DmF). We validate this approach by investigating the well characterized annealing of the HIV-1 (+)/(-) Primer Binding Sequences (PBS) promoted by a HIV-1 nucleocapsid peptide. The TRF-DmF set-up enables resolving the time evolution of sub-populations of reacting species and reveals an early intermediate with a ∼50 ps donor fluorescence lifetime never identified so far. TRF-DmF also favorably compares with single molecule experiments, offering an accurate control of concentrations with no upper limit, no need to graft one partner on a surface and no photobleaching issues.
Reference: Kinetics of protein-assisted nucleic acid interconversion monitored by transient time resolved fluorescence in microfluidic droplets. Grytsyk N, Cianfarani D, Crégut O, Richert L, Boudier C, Humbert N, Didier P, Mély Y, Léonard J.
Nucleic Acids Res. 2021 Aug 27;gkab687. DOI: 10.1093/nar/gkab687